This term is usually applied to bacteria that are not killed by antibiotics. Any population of bacteria, assumed to be all of the same type (e.g., Escherichia coli, or E. coli for short), has a certain genetic variability. If a certain condition (e.g., an antibiotic at a certain concentration) is imposed on the entire population, some individuals may succumb almost immediately, whereas others may survive. The “survival phenotype” may be directly related to the genotype — the surviving bacteria were able to mobilize genetic resources in order to synthesize enzymes that destroyed the antibiotic, or synthesize transporter proteins able to pump the toxin across the membrane and cell wall of the bacteria, thereby eliminating it.
According to the principle of natural selection or “survival of the fittest” those bacteria lacking the survival phenotype will die. The antibiotic will do its work. It may have been hard work for the survivors — they may have had to concentrate their resources in the synthesis of the special proteins for combating the antibiotic — but they will hone their skills and each generation will do it better. They will also become more numerous, because they no longer have to compete with their peers that lack the survival proteins.
Continued, or repeated,* application of the antibiotic will accelerate the emergence of the resistant subtypes, because each successive generation will enjoy the differential advantage due to the antibiotic.
In theory, it would be possible to apply an antibiotic so powerful, or at such a high concentration, so as to kill every single bacterium. This is not done because such high concentrations would harm the human patient.
Hence, the development of resistance is inevitable. Even one bacterium that survives treatment might be sufficient to beget resistant offspring. Sometimes impressive figures are quoted for antibiotics — such as “kills 99.999%”.** There will still be ten survivors out of a million bacteria — which is actually a very small number. If all their peers are dead, they will then have plenty of room to grow and colonize whatever site looks most favourable.
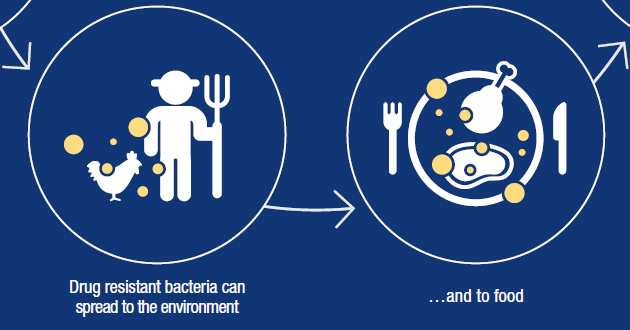
Bacteria can rather freely exchange DNA with other bacteria, even those of different types. They can also mutate rather rapidly. For both these reasons, their genotype, hence phenotype, can evolve rather rapidly if they are pressured to do so by an existential threat. Antibiotic resistance is therefore unavoidable, and it cannot be contained geographically, given the ease with which bacteria can spread.***
* The emergence and growth of antimicrobial resistance is promoted by liberal use by GPs and in hospitals, over-the-counter sales (typical in countries with a large generic antibiotic manufacturing industry), and its use in animal husbandry as a growth promoter and to counteract poor hygiene in animal housing.
** While antimicrobial resistance most typically refers to antibiotics (penicillin and its successors), resistance to disinfectants can also develop.